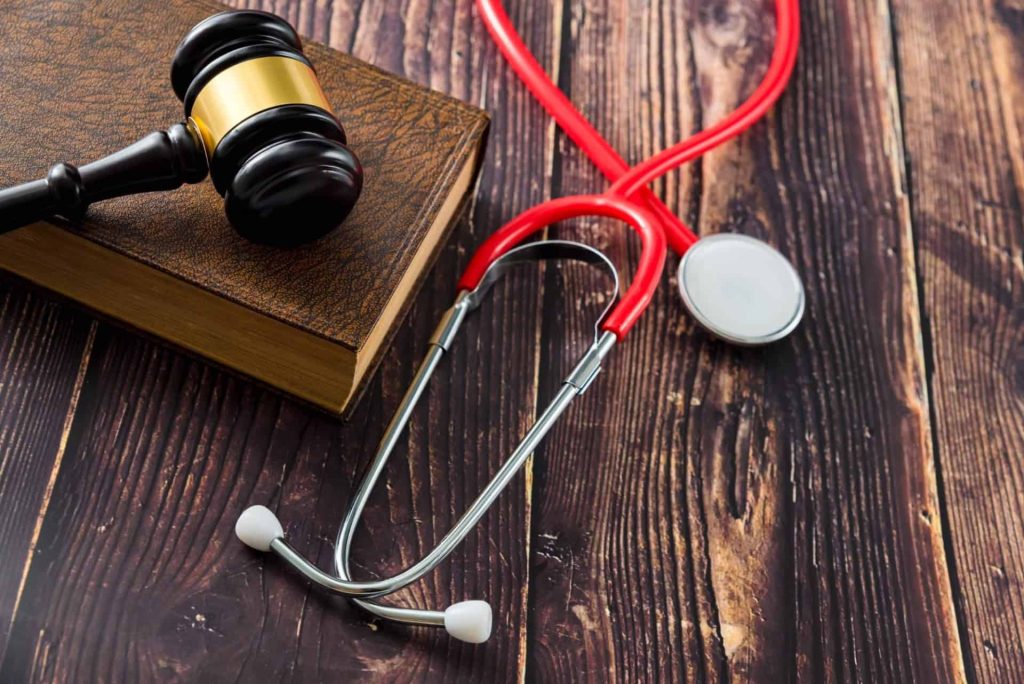
Defective Medical Devices Lawsuits in California
The field of defective medical device litigation in California is governed by the state’s rigorous product liability laws, but with critical exceptions and nuances specific to the healthcare industry.
When a patient is harmed by a faulty implant, surgical tool, diagnostic machine, or any other device, the legal path involves navigating the strict liability doctrine, federal regulatory hurdles, and complex causation standards.
This article details the legal theories, unique defenses, and litigation challenges specific to recovering compensation for injuries caused by medical devices in California.
The Foundation of Medical Device Liability
The central legal principle governing claims is Product Liability in California: Design, Manufacturing, and Warning Defects.
Unlike ordinary personal injury cases, product liability focuses on the inherent flaw in the device itself, rather than the conduct of the person who made it.
Under California Civil Code § 1714.45, liability rules include strict liability for manufacturers and distributors, but also carve out important exceptions.
To succeed in a medical device lawsuit, a plaintiff must prove the device was defective under one of three categories:
1. Manufacturing Defect: Strict Liability Applies
This claim alleges a flaw occurred during the device’s assembly or production, causing the specific item to differ from its intended design (e.g., an implant was contaminated, a suture was cut incorrectly, or a component was improperly soldered).
For manufacturing defects, California’s standard of strict liability applies directly, holding the entire chain of distribution responsible without the need to prove negligence.
2. Design Defect: The Comment K Exemption (A Critical Hurdle)
Unlike standard consumer products, most prescription medical devices and pharmaceutical products are afforded protection from strict liability for design defects under Restatement Second of Torts, Section 402A, Comment k.
California law recognizes that “unavoidably unsafe products” (like many life-saving medical devices) should not be subject to the typical design defect tests, provided they are properly prepared and accompanied by adequate warnings.
3. Failure to Warn (Marketing Defect): The Learned Intermediary Doctrine
A device that is perfectly designed and manufactured may still be legally defective if the manufacturer fails to provide adequate warnings about known or knowable risks.
This marketing defect claim is governed by the Learned Intermediary Doctrine, which holds that the manufacturer’s duty to warn generally runs not to the patient, but to the prescribing physician or surgeon.
Intersection with Healthcare and Workplace Law
A significant area of complexity in these cases involves the overlap between different legal fields, summarized under the umbrella of Product & Workplace Injury Claims in California.
When an injury occurs, a patient must understand the difference between a claim against their doctor and a claim against the device maker.
This distinction addresses the concepts of Workplace Injuries in California: Workers’ Comp vs. Personal Injury Lawsuits and Third-Party Claims in California Workplace Injury Cases.
-
Malpractice vs. Product Liability: A claim against the doctor or hospital for an error in judgment or care is medical malpractice. A claim against the manufacturer for a defect in the device itself is product liability.
-
Third-Party Claims: The manufacturer and distributor of the device are considered third parties outside the direct patient-physician or employer-employee relationship. Filing a lawsuit against this third party manufacturer is essential because it allows the injured patient to recover full damages for pain, suffering, and emotional distress, which are often limited in malpractice claims due to California's MICRA cap on non-economic damages.
Federal Preemption: The Ultimate Defense for Manufacturers
Medical device manufacturers, particularly those creating high-risk, life-sustaining devices, frequently invoke the defense of Federal Preemption, based on the Medical Device Amendments (MDA) of 1976.
The Riegel Rule: The U.S. Supreme Court's ruling in Riegel v. Medtronic established that state law product liability claims related to the design or labeling of an FDA-approved Class III device that underwent the Pre-Market Approval (PMA) process are generally preempted (barred) by federal law.
The Exception: This preemption generally does not apply to a claim based on a manufacturing defect or a failure to warn that violates the specific, federally-approved specifications.
Oversight and Safety in California
In addition to federal rules, California operates its own Medical Device Safety Program under the Department of Public Health.
The program adds a state-level safeguard by monitoring device safety, inspecting manufacturers and distributors, and investigating adverse events reported by hospitals or patients.
It also enforces compliance with labeling rules, recall orders, and quality standards, often working in tandem with federal regulators.
For injured patients, records from CDPH investigations or safety notices can become powerful evidence in proving that a device was defective or that a manufacturer failed to act on known risks.
Mass Torts and California Class Actions for Defective Products
For devices that harm thousands of patients, litigation is often consolidated. While medical device litigation rarely proceeds as a true California Class Action, where all plaintiffs are grouped into a single class, most are handled as Mass Torts (or Multi-District Litigation, MDL).
This process groups similar individual lawsuits (e.g., all hip implant failures) into one federal court for coordinated pre-trial discovery, but the cases retain their individual nature, allowing for tailored damage claims specific to each patient.
The Statute of Limitations and Discovery Rule
For any defective medical device case in California, the statute of limitations is a strict deadline. Most product liability lawsuits must be filed within two years of the date the injury occurred or was discovered.
Given the latent nature of many device complications, the "discovery rule" is critical: the two-year clock starts on the day the patient knew, or through reasonable diligence should have known, that their injury was caused by the device's defect, not simply the natural progression of their illness.
The California court system even provides an official Products Liability Cause of Action Form (PLD-PI-001), which is frequently used to structure claims when filing suit.
Balancing Patient Rights and Innovation
Successfully navigating a Defective Medical Device Lawsuit in California requires a deep understanding of strict product liability principles, the Comment k exemption, and the powerful defense of Federal Preemption.
These doctrines shape not only how cases are argued, but also the types of evidence and expert testimony needed to succeed.
The law seeks to strike a careful balance: ensuring patients can recover when defects cause harm, while at the same time protecting manufacturers who deliver life-saving technologies from being unfairly penalized.
This tension means that every case is fact-specific, hinging on whether the device’s risks were unavoidable, properly disclosed, and manufactured according to approved standards.
For patients, this often translates into a challenging legal journey that requires persistence and skilled representation. For device makers, it underscores the importance of transparency, compliance, and continuous monitoring.
Ultimately, the system aims to encourage innovation without sacrificing accountability, so that Californians can benefit from advanced medical technology with greater confidence in its safety.
People Also Ask
What is the statute of limitations for defective medical device lawsuits in California?
Most product liability lawsuits must be filed within two years of when the injury occurred or was discovered. California applies the “discovery rule,” meaning the deadline begins when the patient knew—or reasonably should have known—that the device caused the injury.
Can you sue a doctor and the device manufacturer at the same time?
Yes. A claim against the doctor would be medical malpractice, while a claim against the manufacturer is product liability. The two are separate legal theories and may be pursued simultaneously.
What defenses do medical device manufacturers use in California?
Manufacturers often raise the defense of federal preemption under the Medical Device Amendments of 1976. They may also argue that the device’s risks were properly disclosed under the Comment k exemption or that the physician was adequately warned under the Learned Intermediary Doctrine.
Are defective medical device cases handled as class actions in California?
Not usually. Most are managed as mass torts or multi-district litigation (MDL), which consolidate pretrial proceedings but allow each injured patient to pursue an individual damages claim.