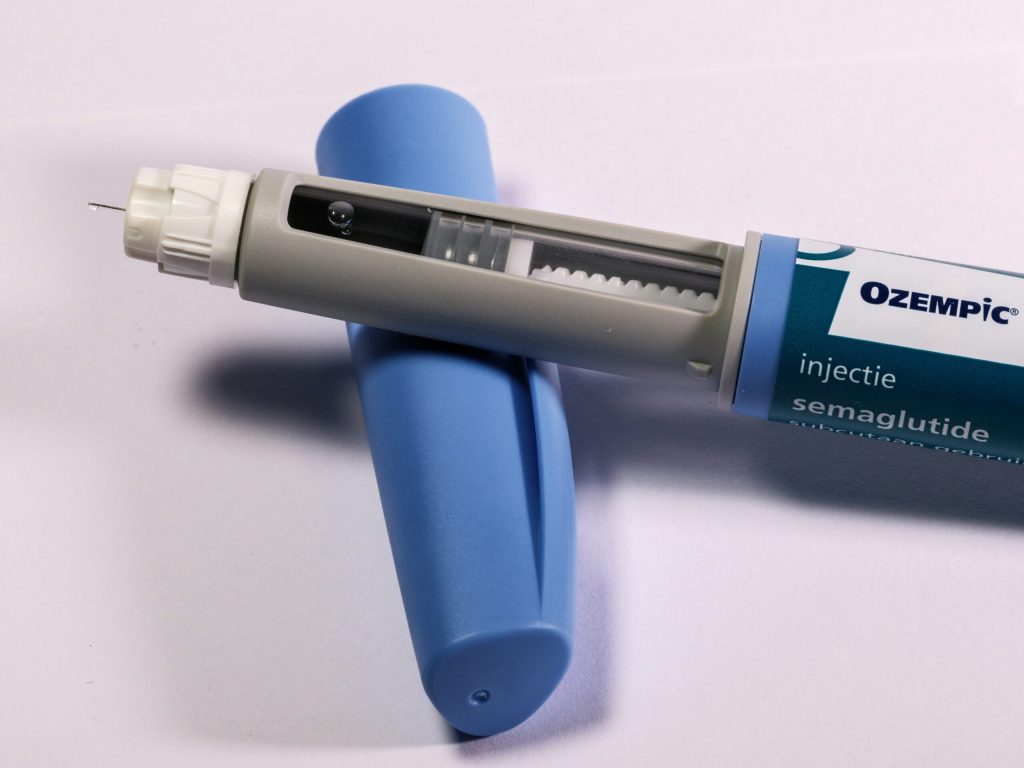
Ozempic Lawsuits: The Legal Risks Behind America’s Weight-Loss Craze
When headlines turned Ozempic from a diabetes medication into Hollywood’s favorite slimming secret, few anticipated the legal storm that would follow.
What began as a medical breakthrough has become one of the fastest-growing mass-tort litigations in the United States, testing how far the law can stretch to cover a “miracle drug” whose side effects are only now being understood.
From Miracle to MDL
Ozempic’s active ingredient, semaglutide, was approved for type 2 diabetes, not vanity.
Yet as off-label prescriptions exploded, reports of gastroparesis (stomach paralysis), intestinal blockages, and even vision loss began surfacing.
Those claims now sit before Judge Karen Spencer Marston in the Eastern District of Pennsylvania, under MDL No. 3094: In re GLP-1 Receptor Agonists Products Liability Litigation.
Early case-management orders have already raised the evidentiary bar: plaintiffs alleging gastroparesis must support their claims with objective gastric-emptying tests, not anecdotal symptoms.
Novo Nordisk and Eli Lilly - the principal defendants deny wrongdoing, arguing that warnings were FDA-approved and that some injuries stem from counterfeit or compounded products outside their control.
At the same time, the FDA seized hundreds of fake Ozempic pens from U.S. distributors in April 2025 and later warned consumers to avoid unverified semaglutide sources.
Enforcement discretion for compounded GLP-1 versions is being phased out, closing the door on many pandemic-era loopholes.
What Doctors Are Actually Saying
Endocrinologists broadly agree that GLP-1 drugs work sometimes dramatically. A 2024 Nature Medicine trial found sustained weight reduction and cardiovascular benefit at 104 weeks.
Yet researchers at the University of Chicago caution that semaglutide is “a powerful tool, but not a magic fix.”
Still, risks remain. Harvard Health notes that rapid fat loss can lead to sagging skin and fatigue, while gastrointestinal side effects like nausea are common.
Anesthesiologists revised their guidance in late 2024, now advising that most patients can continue GLP-1 therapybefore elective surgery, though individual risk assessments are encouraged.
Other findings are newer. Ophthalmology journals have reported a possible association between semaglutide and non-arteritic anterior ischemic optic neuropathy (NAION), a rare cause of sudden vision loss, but emphasize that causation has not been established.
Radiology teams, meanwhile, warn that GLP-1 drugs may alter PET/CT uptake - a discovery presented at the 2025 European Association of Nuclear Medicine Congress, suggesting caution in interpreting cancer scans.
The Celebrity Effect: From Admissions to Irony
Public fascination has turned Ozempic into both a status symbol and a punchline. Sharon Osbourne admitted losing over 40 pounds before warning she had gone “too far.”
Amy Schumer said she lost 30 pounds but quit after “being bedridden with nausea,” later accusing Hollywood of “lying” about its use. Stephen Fry described vomiting “five times a day” before stopping entirely.
And then there’s Katy Perry, who reportedly mocked the speculation by giving guests novelty syringes labeled “OzempiKP” at her 40th birthday party.

Singer Katy Perry pictured at a 2025 Los Angeles event, where she later joked about Ozempic rumors by handing out novelty “OzempiKP” syringes at her birthday celebration.
Friends said that her slimmer figure came from strict diet and exercise with fiancé Orlando Bloom’s support, not injections.
The gesture half satire, half self-awareness captures how pop culture’s obsession fuels demand while blurring medical boundaries.
For regulators and litigators alike, celebrity visibility matters.
FDA officials have already warned that social-media hype and telehealth advertising can mislead consumers, and such promotions are now subject to tighter scrutiny under the agency’s 2025 direct-to-consumer enforcement initiative.
The Law in Focus
Personal-injury law governs pharmaceutical harm through overlapping doctrines: failure to warn, design defect, manufacturing defect, and negligent marketing.
Plaintiffs in the Ozempic MDL allege that Novo Nordisk and Eli Lilly under-disclosed the risk of prolonged gastric paralysis and NAION, while over-promoting aesthetic benefits.
Defendants counter that FDA-approved labeling already listed gastrointestinal risks and that state-law claims are pre-empted by federal regulation.
The learned-intermediary doctrine, which holds that manufacturers must warn doctors, not patients directly features prominently in early motions.
Courts are also testing product-identification standards, since counterfeit or compounded formulations complicate proof of origin.
Punitive-damage claims hinge on marketing intent: did the companies, or affiliated telehealth promoters, glamorize the drug beyond its clinical use?
With more than a thousand suits pending and bellwether trials likely in 2026, this MDL could define how far liability extends for future “lifestyle” pharmaceuticals.
The FDA’s ongoing counterfeit investigations and new advertising enforcement actions will shape discovery, causation arguments, and even settlement value.
A Broader Reckoning
Ozempic’s story has evolved far beyond the realm of weight loss. What began as a medical innovation has become a test case for how the law adapts to scientific progress and how society negotiates the line between personal responsibility and corporate accountability.
The same drug that reshaped countless lives is now forcing courts to revisit long-standing questions about product safety, marketing ethics, and informed consent in the era of social media influence.
The unprecedented surge in off-label use has blurred distinctions between therapeutic treatment and lifestyle enhancement, leaving regulators, physicians, and attorneys to sort out where medical innovation ends and consumer harm begins.
At the heart of this reckoning lies a deeper cultural issue. America’s obsession with rapid results be it through diet trends, cosmetic procedures, or pharmaceutical shortcuts, has created fertile ground for both innovation and exploitation.
The ongoing multidistrict litigation will determine more than financial liability; it will help define how future drugs are marketed, prescribed, and monitored once they escape the laboratory and enter the public imagination.
Whether the MDL concludes with sweeping settlements or a narrow judicial precedent, one truth has already emerged: in the pursuit of thinness and control, the fine print now carries as much weight as the scale itself.
People Also Ask
What are the main lawsuits against Ozempic about?
Most Ozempic lawsuits claim the drug caused serious gastrointestinal injuries such as gastroparesis (stomach paralysis), intestinal blockages, and severe nausea or vomiting. Some cases also allege vision loss linked to a condition called NAION (non-arteritic anterior ischemic optic neuropathy).
Who is leading the Ozempic multidistrict litigation (MDL)?
All federal Ozempic and GLP-1 drug injury cases are consolidated under MDL No. 3094 in the Eastern District of Pennsylvania, overseen by Judge Karen Spencer Marston. The MDL coordinates discovery and pre-trial rulings for claims against Novo Nordisk and Eli Lilly.
Has the FDA taken action over Ozempic safety?
Yes. In April 2025, the U.S. Food and Drug Administration seized counterfeit Ozempic pens and issued warnings about unapproved or compounded semaglutide products. The agency also tightened advertising oversight for telehealth providers promoting GLP-1 drugs.
Can patients sue for injuries from compounded or counterfeit Ozempic?
They can, but these cases are more complex. Plaintiffs must prove the specific product source—whether it was authentic, compounded, or counterfeit—to establish liability. Claims may extend beyond the manufacturer to pharmacies or distributors.
What side effects are doctors most concerned about?
Medical experts acknowledge Ozempic’s effectiveness for weight loss and diabetes management but warn of nausea, dehydration, loss of muscle mass, delayed gastric emptying, and rapid rebound weight gain if treatment stops abruptly.
Have any celebrities admitted using Ozempic?
Public figures such as Sharon Osbourne, Amy Schumer, and Stephen Fry have discussed using or discontinuing the drug, citing side effects or ethical concerns. Katy Perry, meanwhile, mocked the speculation by handing out novelty “OzempiKP” syringes at her 40th birthday party.
What happens next in the Ozempic lawsuits?
The MDL is still in early discovery. Bellwether trials could begin in 2026, which will help determine the strength of the claims and shape future settlement negotiations.