Medical Malpractice & Product Liability: Depo-Provera and Gardasil
The proliferation of advanced medical technologies and consumer products has increased both their utility and the legal complexity surrounding their use.
In response, the legal principles of medical and product liability provide a critical framework for assigning accountability and seeking redress for injuries caused by professional negligence or product defects.
This article will analyze the core distinctions within this area of jurisprudence and examine its application in two landmark cases that are currently shaping the legal landscape.
The Foundations of Liability
The first thing to understand is the fundamental difference between these two legal avenues. We are, at our core, talking about two distinct types of failures.
A medical malpractice claim is an intensely personal one, centering on a betrayal of professional duty. It alleges that a healthcare provider be it a doctor, nurse, or hospital, failed to meet the accepted standard of care, causing a patient's injury or death.
The legal question isn't simply "did something go wrong?" but rather, "did the provider's conduct fall below what a reasonably competent professional would have done?"
Proving this requires peeling back the layers of a complex medical scenario, often relying on expert testimony to establish the prevailing standard and how the defendant failed to meet it. It's a heavy burden, but a necessary one to ensure justice for preventable harm.
Product liability, by contrast, looks beyond a single professional’s actions to the product itself. The legal focus shifts from the provider to the entire chain of distribution from the manufacturer who designed it to the retailer who sold it.
The core principle is that a defective product is inherently dangerous, and a plaintiff has several legal avenues to prove it.
Identifying the Flaw
So, how does the law define a defective product? The reality is, it depends on where the flaw originates.
First, there's the manufacturing defect, the easiest to spot and prove. This is when the product you received is flawed because of an error during its creation, making it different and more dangerous than its intended design.
Think of a baby food jar with a stray shard of glass inside, or a surgical implant that wasn’t properly sterilized.
The legal theory of strict liability is particularly potent here; it holds the manufacturer responsible regardless of whether they were negligent. All a plaintiff has to show is that the product was defective when it left the manufacturer's control and that this defect caused the injury.
Second, the design defect, which is far more challenging to litigate. Here, the flaw isn't in a single item, but in the entire product line. The design itself is inherently unsafe, even if every unit was produced perfectly.
To win this argument, a plaintiff must demonstrate that a safer, economically feasible alternative existed at the time of manufacture. It requires a detailed analysis a "risk-utility test" that weighs the product’s dangers against its benefits.
The infamous Ford Pinto case, where the car’s fuel tank was dangerously positioned, is the classic example of a disastrous design flaw.
Finally, the warning defect, or a "failure to warn," is an increasingly common basis for pharmaceutical lawsuits.
This occurs when a product has a non-obvious danger that the manufacturer knew or should have known about, but failed to adequately warn consumers. Proving this requires showing that a reasonable warning would have changed a consumer's behavior and prevented the injury. This is the central legal argument in a torrent of ongoing litigations.
This liability can extend across the entire supply chain. A plaintiff isn't just limited to suing the original manufacturer.
They can also seek compensation from distributors, wholesalers, and even the retailer who sold the defective product. This is a crucial advantage for plaintiffs, as it increases the chances of finding a financially solvent party to compensate for damages.
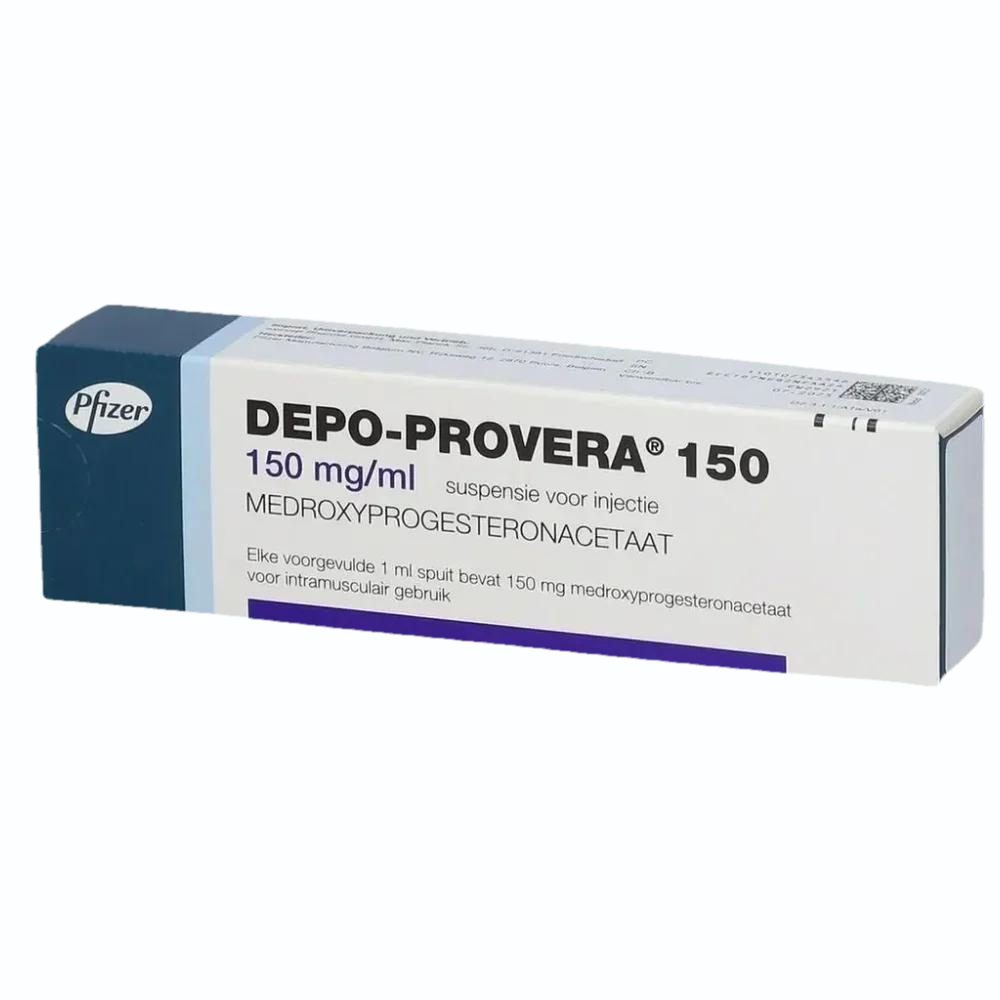
Case Study 1: The Depo-Provera Brain Tumor Litigation
The ongoing legal storm surrounding the injectable contraceptive Depo-Provera provides a stark reminder of the long-term consequences of a potential failure to warn.
What began as quiet concerns about side effects has now swelled into a wave of lawsuits, with women alleging that the injectable contraceptive left them with life-altering brain tumours known as meningiomas.
At the heart of the litigation lies a simple but devastating question: did Pfizer know about these risks and choose not to say anything? Plaintiffs argue that the company had data for decades suggesting a potential danger.
“Patients were denied the ability to make an informed choice - a fundamental right in medical treatment.” said Ellen Relkin, one of the attorneys.
Pfizer has pushed back strongly. In an August 2025 filing, the company claimed it sought to add a tumour warning in 2023 but was blocked by the U.S. Food and Drug Administration, which concluded the evidence was not conclusive enough.
The company insists that because the FDA rejected the label change, federal law prevents plaintiffs from pursuing state-law failure-to-warn claims.
For the hundreds of women whose cases have now been bundled together in a Multidistrict Litigation in Florida, the legal wrangling feels personal. Many say they spent years trusting their doctors, only to later discover that a medication they believed was safe might have changed their lives forever.
“The law makes clear drug manufacturers like Pfizer are responsible for providing proper warnings to patients and doctors,” said attorney Bryan Aylstock in a recent filing.
The first bellwether trials will test how juries respond to these arguments. A win for Pfizer could blunt the wave of claims; a win for plaintiffs could set the stage for massive settlements and force new safety warnings. Either way, the outcome will ripple far beyond the courtroom.
Case Study 2: The Gardasil HPV Vaccine Litigation
Perhaps no other product liability case has captured the public’s attention quite like the litigation against Merck over its HPV vaccine, Gardasil.
The lawsuits, particularly those spearheaded by firms like Wisner Baum, are highly contentious, alleging that the vaccine causes severe autoimmune and neurological injuries, including Postural Orthostatic Tachycardia Syndrome (POTS) and Chronic Fatigue Syndrome.
Plaintiffs claim that Merck overhyped the vaccine’s safety and efficacy while downplaying its potential for serious side effects. These legal arguments are very similar to the Depo-Provera case, focusing on the "failure to warn."
The case has also raised ethical questions for the legal community. Financial disclosures from Robert F. Kennedy Jr. indicate he received millions in referral fees from the law firm, highlighting how deeply intertwined prominent public figures can become with active litigation.
In a signed ethics agreement submitted to the U.S. Office of Government Ethics, Robert F. Kennedy Jr. clarified his position, stating that he was "entitled to receive 10% of fees awarded in contingency fee cases referred to the firm."
He also noted that he was "not trying these cases" and would not "provide representational services" in connection with them.
The public nature of these filings fueled a broader debate about whether an individual could potentially profit from vaccine litigation while also regulating drugmakers and exercising authority over federal vaccine policy.
The Future of Liability
The fields of medical and product liability are continuously evolving, driven by scientific innovation and the relentless pursuit of justice.
The rise of AI-driven medical devices, for example, presents a new frontier for liability, raising complex questions about where responsibility lies when an algorithm makes a diagnostic error.
The legal system, though slow, serves as a vital mechanism for holding corporations and professionals accountable.
Lawsuits, even those that do not result in a verdict, often compel companies to change their practices, update warnings, or redesign products, ultimately leading to a safer marketplace for everyone.
People Also Ask
What is the difference between medical malpractice and product liability?
Medical malpractice involves negligence by a healthcare provider who fails to meet the accepted standard of care. Product liability focuses on defective products, whether due to design, manufacturing, or failure to warn, regardless of a provider’s conduct.
Can you sue for both medical malpractice and product liability in the same case?
Yes. If a patient is harmed by both a provider’s negligence and a defective medical product, they may pursue claims under both legal theories, though the standards of proof differ.
What are examples of major product liability cases in healthcare?
Notable cases include the Depo-Provera lawsuits, alleging a failure to warn about the risk of brain tumors, and the Gardasil lawsuits, claiming the HPV vaccine caused severe autoimmune and neurological conditions.
How does strict liability apply in product defect cases?
Under strict liability, a manufacturer may be held responsible for injuries caused by a defective product, even if they were not negligent. Plaintiffs only need to show the defect existed and directly caused harm.
What role does failure to warn play in pharmaceutical lawsuits?
Failure to warn occurs when a company knew or should have known about risks but failed to provide adequate warnings. This is central to cases like Depo-Provera and Gardasil, where plaintiffs argue proper warnings would have prevented harm.
Why are multidistrict litigations (MDLs) used in drug liability cases?
MDLs consolidate many individual lawsuits into one court for efficiency in pretrial proceedings. Bellwether trials in MDLs often shape settlement negotiations and influence the outcomes of related cases.
How might new medical technologies like AI affect liability law?
AI-driven devices raise complex questions about who is responsible for errors - the developer, the healthcare provider, or the institution. This is expected to be a major frontier in medical and product liability law.
More Articles